Verisense Blog
In this series of blog posts, our leading researchers and experts will be posting insights on the Verisense platform, while also delving into wider industry topics into the use of wearable sensors in Clinical Trials.
Blog 22 - Personalizing Healthcare with Digital Technologies
Verisense Health recently hosted a webinar delving into how personalizing digital biomarkers and health protocols is crucial for harnessing the full potential of digital health technologies.
Blog 21 - Adding Wearable Sensors to Clinical Trial Protocols
This blog aims to give guidance on how the Verisense IMU sensor for activity and sleep can be seamlessly integrated into a trial protocol with minimal burden on sponsors, sites and participants.
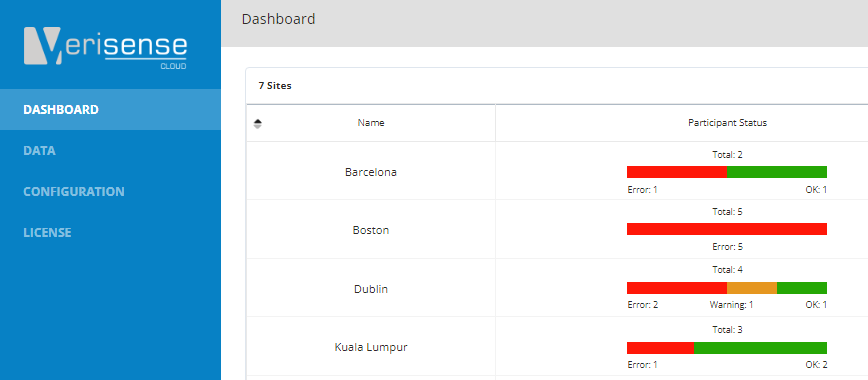
Blog 20 - Verisense Web Portal Explained
Blog 20 gives a brief, high level insight into the user interface of the Verisense web portal. The portal was designed specially to allow for quick and easy deployment and management of wearable sensors in a clinical trial.
Blog 19 - Raw Data vs Processed Data: What It Means for Digital Health
Clinical research demands higher-quality data than most commonly available wearables can provide. Blog 19 delves into shortcomings of sensors that are widely available, advantages that more sophisticated sensors can offer, and problems that remain to be resolved.
Blog 18 - Regulatory Standards & Data Protection
Our first blog of 2022 gives a brief insight into the regulatory and quality controls employed by Shimmer to ensure we are meeting all ISO13485:2016 standards in the production of the hardware and data capture systems used in studies and trials.
Blog 17 - Monitoring Wear Compliance with Verisense
Blog 17 explains the tools and features available within the Verisense system for monitoring wear compliance among study participants.
Blog 16 - Self reported outcomes versus sensor generated outcomes
Blog 16 outlines how wearables can play a huge role in improving the measurements of clinical outcomes.
Blog 15 – New step counter algorithm - Generating step count out of everyday data
Leading on from the release of Verisense’s validation paper according to DiMe’s V3 framework, we dive into the detail of a new step counting algorithm created by Shimmer’s Data scientist, Dr. Matt Patterson, using the GGIR processing package.
Blog 14 - What next for Verisense?
In Blog 14 we preview the next iteration of Verisense - Verisense Pulse+. Adding to it’s existing capabilities, the Verisense platform is now expanding it’s sensing capacity, opening up new avenues and routes to digital endpoints.
Blog 13 - Clinical Trials in Crisis
Blog 13 discusses current difficulties within the Clinical Trials landscape, and looks at how adoption of wearable sensors can be one tool to help address these.
Blog 12 – Verisense End Points and Metrics
The 12th edition of our blog series will focus on the clinical trial end points and metrics that are possible to explore using Shimmer’s Verisense platform.
Blog 11 - Syncing Multiple Sensors
Our Blog series returns for 2021 with a look at how the Verisense platform can easily sync data from multiple sensors to open up a range of different possibilities beyond standard activity and sleep metrics.
Blog 10 - Participant Centric Wearables
Our tenth and final blog for 2020 examines commonly held participant fears about using wearable technology in Clinical Trials, and how Verisense was designed to reduce these and increase Participant compliance.
Blog 9 - The effective use of Sensor-generated measures of health
Our ninth blog post explains the importance of responsible sharing of data and open source data repositories to advance healthcare. Have your say!
Blog 8 - Data Security & Encryption with Verisense
The 8th edition of our blog series focuses on the importance of Data Security and outlines the measures the Verisense platform employs to protect the data of our users.
Blog 7 - What we learned - 4 highlights from DPHARM: Disruptive Innovations 2020
Our seventh blog post outlines our experience at attending The Conference Forum’s DPHARM: Disruptive Innovations US 2020 conference centred on all the innovation that is underway to shake up how the clinical trials industry currently operates.
Blog 6 - Integrating with Verisense
Our sixth blog post explains how data from the Verisense Sensor can be integrated onto external platforms.
Blog 5 - Verisense Algorithm Validation Studies
In Blog 5 we go into detail on how the algorithm Verisense uses to obtain activity and sleep metrics has been validated.